On September 10, Nature Communications published the latest collaborative research on Au(111)|water-in-salt interface structure between the group of Professor Feng from HUST and Professor Lian from Emory University. The article is titled “Unconventional interfacial water structure of highly concentrated aqueous electrolytes at negative electrode polarizations”
With the rapidly increasing demand for renewable energy and mobile devices, the development of next-generation electrochemical energy storage systems has become a key objective in energy community. In electrochemical energy devices, the electrolyte, sandwiched between the positive and negative electrodes, is an essential component determining the performance. Aqueous electrolytes have safety advantages over aprotic organic electrolytes, such as non-flammability and low toxicity. However, conventional aqueous electrolytes are limited by their narrow electrochemical window of 1.23 V due to the water decomposition. This limitation can be overcome in highly concentrated aqueous electrolytes, also known as “water-in-salt” (WiS) electrolytes which have been shown to significantly widen the electrochemical stability window to over 3.0 V, delivering impressive performance in Li-ion batteries, supercapacitors, and CO2 reduction reactions. However, the physicochemical interactions at the electrical double layer (EDL) between the WiS electrolyte and the electrode are not well understood.
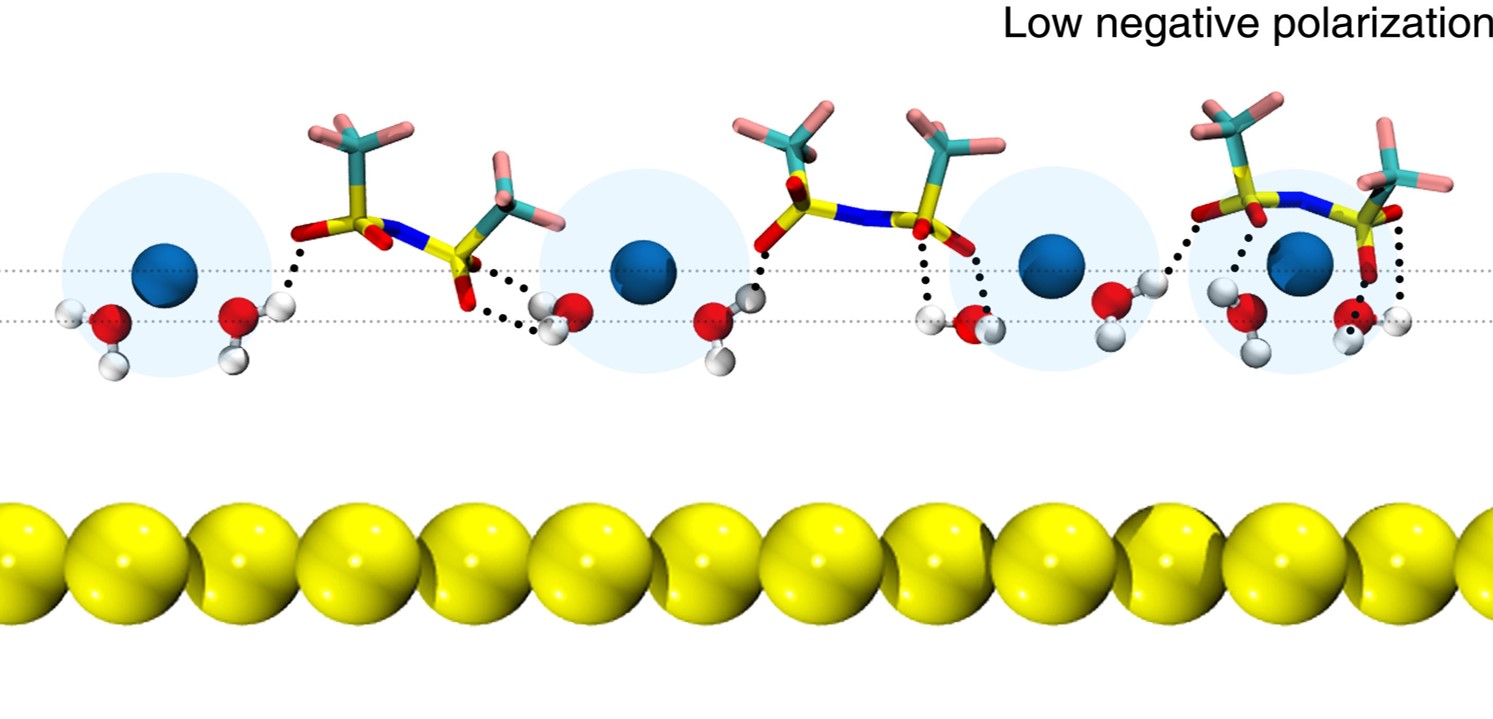
Herein, the potential-dependent EDL structure of highly concentrated LiTFSI aqueous electrolytes (21 m LiTFSI) on an Au(111) electrode was studied at electrode polarizations by the combination of in situ electrochemical shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) and molecular dynamics (MD) simulations. It was revealed that most interfacial water molecules are bound with Li+, showing zero, one, or two hydrogen bonds to feature three OH stretching bands. The accumulation of Li+ on the electrode surface, at large negative polarizations, reduces the interfacial field to induce an unusual “hydrogen-up” structure of interfacial water and blue shift of the OH stretching frequencies, quantitatively differing from lower concentration aqueous electrolytes. This atomistic understanding of the double-layer structure provides key insights into the design of high-performance aqueous electrolytes for electrical energy storage.
Chao-Yu Li (a postdoctoral at Emory University) and Ming Chen (a postdoctoral at State Key Laboratory of Coal Combustion, HUST) contribute equally to this work. The corresponding authors are Prof. Feng and Prof. Lian. Ming Chen and Prof. Feng are supported by the National Natural Science Foundation of China (52106090 and 52161135104), Hubei Provincial Natural Science Foundation of China (2020CFA093), and the Program for HUST Academic Frontier Youth Team.
This research article can be found in https://www.nature.com/articles/s41467-022-33129-8.