Room temperature ionic liquids (ILs), with unique properties including excellent thermal stability, nonflammability and especially wide electrochemical windows, are an emerging class of candidates for electrochemical energy storage (EES) devices, such as supercapacitors, batteries and solar cells. However, owing to their hygroscopic nature, ILs can spontaneously adsorb water from the humid environment, regardless of their hydrophilicity/hydrophobicity. It has been reported, by our previous work (Nature Communications 2018, 9, 5222, https://doi.org/10.1038/s41467-018-07674-0), the water electrosorption on the electrode is governed by the working voltage and the association of water molecules with their neighbors (including surface charges, electrode materials and IL ions), and due to the strong interaction with anions, water molecules are excluded from the negatively charged electrode with hydrophilic ILs. However, it is still an unaddressed issue that, for hydrophobic ILs that have been widely used as electrolytes, they become humid when exposed to the atmosphere, and unfortunately, their water much favors adsorption on both negatively and positively charged electrodes. To avoid compromising their wide electrochemical windows, therefore, it is of great importance to minimize surface-adsorbed water and its impact.
To address such an issue, in this study, analogous to the “water-in-salt” electrolyte, we performed molecular dynamics (MD) simulations to investigate the effect of adding Li salt in humid hydrophobic ILs on the ion and water distributions near electrodes. Results disclose that, water is excluded from the electrode surface and the activity of water remaining in the interfacial region is reduced by adding salt, which could dramatically enhance the electrochemical window of humid ILs. This conclusion is verified by electrochemical cyclic voltammetry measurements on carbon electrodes in contact with humid ILs and salt-in-humid ILs.
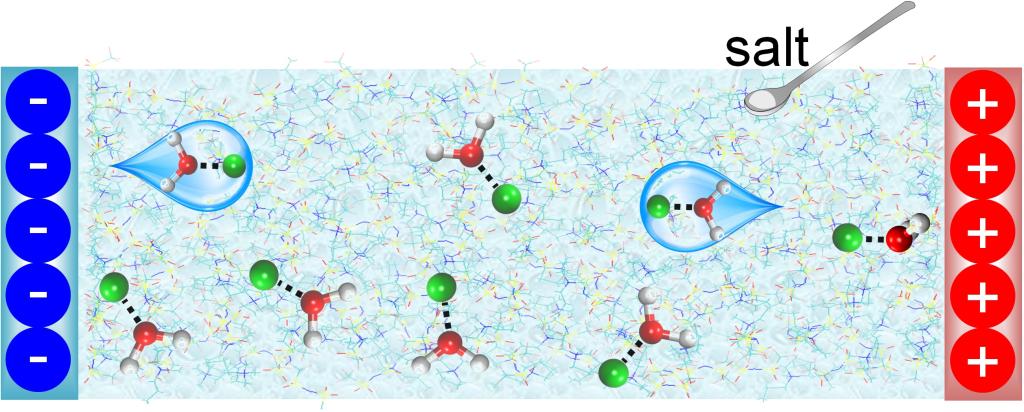
We understand these observations by MD simulation and DFT calculations. The water is found to have stronger association with Li+ than IL ions. Therefore, since Li+ ions mostly prefer to stay away from the electrode surface, water tends to be pulled away from the electrode under positive and moderately negative polarizations, extremely reducing the water in the interfacial region. For water molecules still adsorbed on the electrode surface, most of them are bound with Li+, resulting in significantly decreased activity, since the strength of O-H bond in bound water is increased due to the association with Li+ and thus more energy is needed to split the O-H bond. Meanwhile, the added Li+ modifies the arrangement of adsorbed water, by changing its orientation and atom position, protecting water from electrolysis. The lower HOMO level is a complementary mechanism for the thermodynamic manner to improve electrolyte oxidation stability under positive polarization.
Briefly, our findings reported with the concept of salt-in-humid ILs could extend the comprehension of the preferential species electrosorption, and provide a guideline for minimizing water adsorption and altering the structure and property of adsorbed water.
All the details of our work can be read in our article: Nature Communications 2020, **, ****, which can be downloaded from the link: https://doi.org/10.1038/s41467-020-19469-3